
Day(s)
:
Hour(s)
:
Minute(s)
:
Second(s)
OVERVIEW
HTA Ignite: Real-World, Real-Impact
The ME Pharmacoeconomics & Drug Policy Summit, under the core theme “HTA Ignite: Real-World,Real-Impact,” highlights evidence-based assessments and the development of transparent frameworksfor value-driven healthcare decisions across the Middle East. The event will gather regulators, healtheconomists, payers, providers, and industry leaders to share insights, exchange strategies, and explore innovative approaches for enhancing patient outcomes while maximizing resource efficiency.
The summit will demonstrate how Health Technology Assessment (HTA) harnesses Real-World Data (RWD) to produce actionable Real-World Evidence (RWE) by assessing the clinical, economic, and societal impact of therapies, HTA enables value-based pricing, improves access to advanced treatments, and promotes sustainable healthcare practices. Integrating RWE with HTA ensures that policies are both practical and patient-centered, driving long-term improvements in healthcare delivery across the region.


















Who Attended 2025 Summit
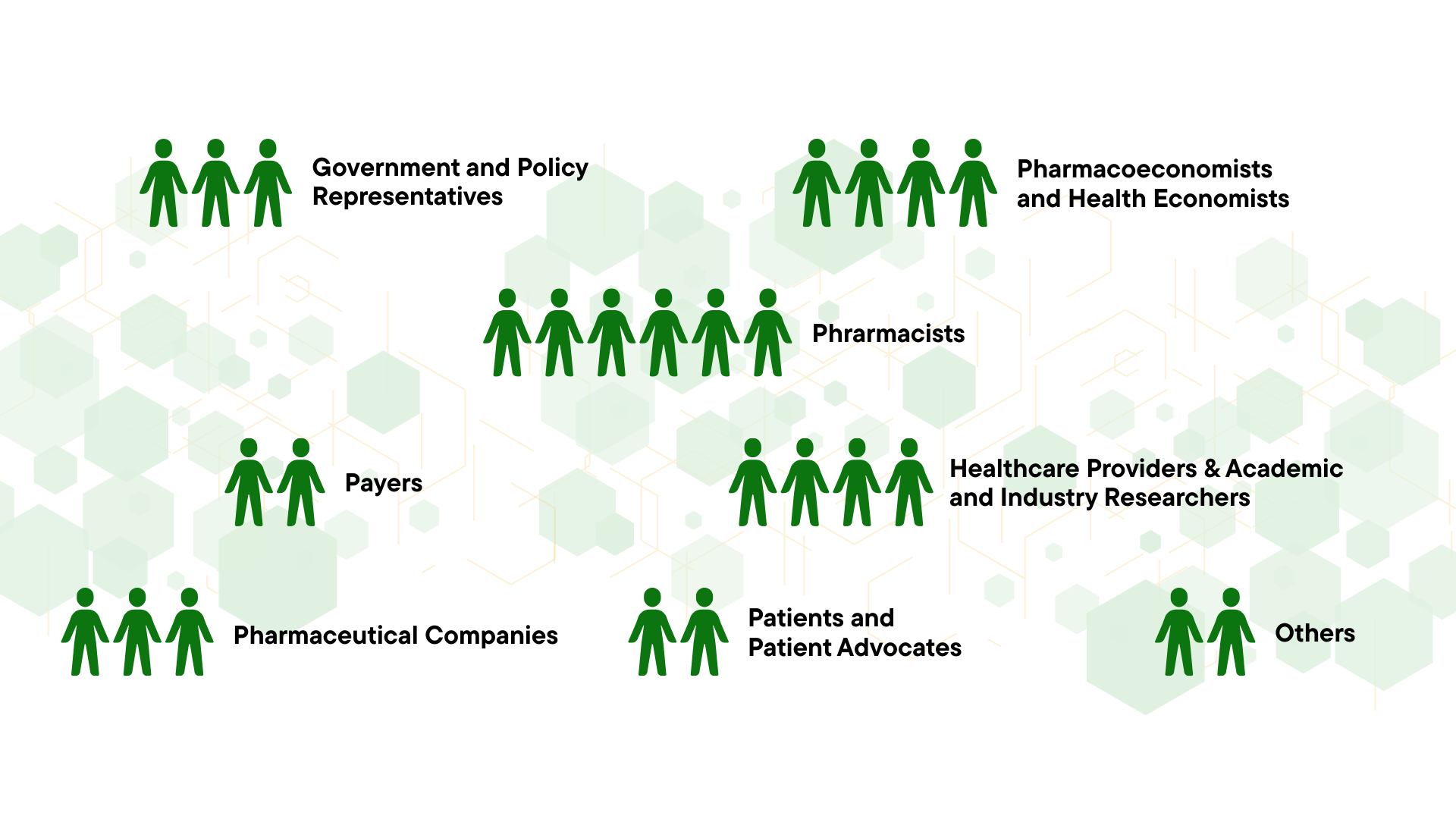
Conference Key Facts

WHO SHOULD ATTEND
- Regulatory authorities
- Pharmacoeconomists
- Health Economists
- Health Technology Assessment Specialists
- Payers & Health Insurance – Drug pricing & Reimbursement Departments
- Healthcare providers
- Pharma Industry: Market access teams
- Pharmacists
- Real-World Evidence (RWE) and Real-World Data (RWD) experts
- HEOR (Health Economics & Outcomes Research) professionals
- Pricing and policy strategists
- National HTA agencies and committees
KEY BENEFITS OF ATTENDING
- Highlight future opportunities and challenges in developing the healthcare and research economy in the GCC countries
- Discuss the novel approaches to value assessment, achieving cost effective healthcare solutions and access to innovative medicines in the GCC
- Understanding the principles of outcome based healthcare and evidence based medication
- Reviewing pharmacoeconomics and outcomes research strategies
- Exploring the real world data, the burden of disease and HTA to make proper assessments and support decision making
Summit chairman

Ahmed Al Jedai
SUMMIT VICE CHAIR

Mohammed Alshennawi
General Director, General Administration of Pharmaceutical Care, Ministry of Health, KSA
ORGANIZING COMMITTEE CHAIR

Wejdan I. Aburas
Director, Drug Policy and Regulation, Senior Cardiology Clinical Pharmacist, Therapeutic Affairs Deputyship, Ministry of Health, KSA
Scientific Committee Chair

Hajer Al-Mudaiheem
Board Member, Saudi Society of Clinical Pharmacy (SSCP), KSA
Strategic Partner


AN INITIATIVE BY

Summit Packages
2-Days Summit Access
Physicians, Regulatory Authorities, Health Economists, Hospital and Community Pharmacists
Pharmaceutical Companies and Vendors




